Development of European HTA: from Vision to EUnetHTA
Michael 2012;9: 147–156
Most European countries are formally building HTA into policy and reimbursement processes. The development of HTA in Europe has been a combination of scientific, political and practical steps in a region of the world that provides specific conditions for that to happen – the European integration and the EU.
The strategic objectives of the European network for HTA (EUnetHTA) which started in 2006 were to promote more effective use of resources, increase HTA input to decision making, strengthen the link between HTA and policy making, and support countries with limited experience in HTA. The most innovative scientific and practical provision has been the HTA Core Model. It enables effective national and transnational production and sharing of HTA results in a common, structured format and represents a wide range of perspectives. Other results were a handbook on HTA capacity building, a toolkit for adapting existing HTA reports to other settings, and a database of studies for additional data collection on new technologies.
In 2011 the EU Research Framework Programme 7 made a call for proposals in new methodologies for HTA based on a study of research needs. This is a major step in bringing HTA into the research agenda in Europe.
A majority of Member States in the European Union (EU) and the European Economic Area (EEA) now have public sector Health Technology Assessment (HTA) agencies that provide information to decision making and policy making at the national or regional and levels (1). Many European countries are formally building HTA into policy, governance, reimbursement, and/or regulatory processes. The development of HTA in Europe has been a unique combination of scientific, political and practical steps taken in a region of the world that provides specific conditions for that to happen – conditions such as the process of European integration and the EU and its Health and Research programmes (2). This article describes the scientific development of, the contemporary needs of a research basis for, and the historical and policy background of a sustainable European network for HTA.
The European Network for Health Technology Assessment – EUnetHTA
During 2006 to 2008, the EUnetHTA Project (www.eunethta.eu) examined the entire process of HTA and its links to policy, addressing several key challenges (e.g., reporting standards, information sharing) for enhancing transnational collaboration. The EUnetHTA Project was established to create an effective and sustainable network for HTA across Europe that could develop and implement practical tools to provide reliable, timely, transparent, and transferable information to HTAs in EU Member States and EEA countries. The strategic objectives of the EUnetHTA Project were to reduce duplication of effort to promote more effective use of resources, increase HTA input to decision making in European countries and the EU, to increase the impact of HTA at all levels of healthcare, strengthen the link between HTA and healthcare policy making in the EU and its Member States, and support countries with limited experience in HTA (3).
EUnetHTA Project Results
The project was organised into a number of work streams with the following themes.
Develop and Pilot Test a Framework and Model for Transnational HTA
The most innovative scientific and practical provision of EUnetHTA until now has been the HTA Core Model (4). It is a novel approach to HTA. During the project a framework and a model were developed for the assessment of medical and surgical interventions and for diagnostic technologies. Nine domains (Health problem and current use; Description and technical characteristics; Safety; Effectiveness; Costs and economic evaluation; Ethical; Organisational; Social; and Legal aspects) based on the EUR-ASSESS Project, (an earlier European project in HTA which identified experts in HTA in different European countries and brought them into the community of HTA researchers in Europe) were included in the model (5). The HTA Core Model enables effective national and transnational production and sharing of HTA results in a common, structured format and represents a wide range of perspectives as reflected by the nine domains listed above. It is currently developed into a platform that enables and encourages genuine transnational HTA collaboration between institutions and individuals in terms of work distribution and maximum utilisation of a common pool of structured HTA information for national HTA reports (4).
Develop and Pilot Test Tools to Adapt HTA Reports to New Contexts
The numerous HTA agencies across Europe each produce their own HTA reports – often on the same topic – which is useful but time consuming and costly. This work stream had the following objectives: (i) Examine and understand the process of adaptation; (ii) Investigate whether the adaptation of HTA reports could be useful to agencies across Europe, and how this might be achieved in practice; (iii) Develop an HTA adaptation toolkit for use by agencies across Europe; (iv) Undertake quality assurance testing of this toolkit; and (v) Develop a glossary of HTA adaptation terms to help reduce misunderstanding of terms used in HTA reports from contexts other than the reader’s own. The toolkit and glossary will be valuable resources when reading HTA reports produced in different contexts and when adapting HTA reports produced in other countries (6). The next steps include web-based implementation and more field-testing within the EUnetHTA Joint Action, which is a European Commission supported three-year project involving a total of 35 government appointed organisations from 24 EU Member States, Norway and Croatia and a large number of regional agencies and non-for-profit organisations that produce or contribute to HTA (see www.EU.netHTA.eu).
Develop and Pilot Test Tools to generate Transnational Evidence for HTA of New technology
Several countries have developed policy frameworks allowing timely access to promising health technologies on the condition that additional evidence is generated (7). Access with evidence generation (AEG) is well known in the context of marketing approval, but is a more recent concept in relation to coverage (i.e., inclusion in standard care, reimbursement by health insurance, etc.). However, an important barrier to evidence generation on new promising technologies in terms of volume and speed is the lack of structured collaboration among HTA agencies across borders. This work stream had the following objectives: (i) Create an overview of known national AEG mechanisms in various countries associated with marketing approvals and funding or coverage decisions; and (ii) Determine the types of structured collaboration that could facilitate evidence generation and to develop a Web-based toolkit to support this. International collaboration is particularly needed to gather a critical mass of high-quality data quickly, while ensuring timely access to promising technologies. The new Web site for sharing information on evidence generation should help managers and policy makers in making robust decisions on the timely adoption of promising health technologies (7).
Support to HTA Capacity Building
The number of HTA agencies in Europe has grown rapidly in recent years. Furthermore, many countries without a formal HTA program are showing increased interest in establishing one. The experiences of countries that have institutionalised HTA could be helpful for other countries. The objectives of this work stream were the following: (i) Define the minimum components related to the scope, structure, process, and visibility of an HTA organisation; (ii) Develop tools for information support to organisations or institutions implementing HTA; and (iii) Produce a handbook. The results were compiled into a Handbook on HTA Capacity Building. In the establishment of a new HTA agency, it is important not only to secure funding, but also to attract trained staff. Multidisciplinary teams composed of a wide range of specialised professionals, researchers, and administrative assistants are the ideal work units needed to produce sound HTA reports. Ultimate success also depends on the quality and relevance of HTA reports, an efficient information dissemination system, and a willingness at the policy level to integrate HTA into decision making. It is also worth taking advantage of the new technologies to promote and become more active in planning dissemination strategies (8).
Inform Decision Makers About Emerging Technologies
Dissemination of an EU-wide newsletter on emerging technologies is a feasible but time-consuming activity, concluded EUnetHTA. An EU-only newsletter based on information from EuroScan International Network was not considered to be the appropriate instrument, and EUnetHTA would avoid duplication of efforts planned by EuroScan. Thus EUnetHTA trusted that the global network EuroScan, a network that exchange information on new and emerging technologies, would carry this out (9).
Stakeholder Involvement
EUnetHTA identified five stakeholder groups at the European level as potentially sharing an interest in EUnetHTA and its products: (i) Policy makers at national and regional levels, (ii) Policy makers at the institutional level, (iii) Patient organisations, (iv) Health care professionals, and (v) Industry. A draft stakeholder policy for the EUnetHTA Collaboration was developed and discussed with stakeholders in a face-to-face meeting in 2008 (10). This lead to the establishment of a Stakeholder Forum comprising payers, providers, patients, and industry as an integral element of the governance and management structure of the Network.
Internal Evaluation
The main outcome from the evaluation of the EUnetHTA Project was that it succeeded in developing tools aimed at providing a common methodology, this being with the intent to establish a suggested standard for conducting and reporting HTA and facilitating increased collaboration between agencies. The participants expressed their belief in a network while maintaining local or national autonomy. The partners expressed a strong belief that the solid base provided by the project would serve as a future network, but were aware of the need for funding and governmental support (11).
Research Trends and Future Priorities in European HTA
An EU DG Research and Innovation Framework 7 project to develop a Health Services Research agenda for Europe provided an overview of health services research related to HTA together with the identification research priorities for HTA from a European perspective (12). Several methods were used: Systematic review of articles indexed with the MeSH term ‘technology assessment’ in PubMed 1999–2009; online survey among experts, and conference workshop discussions. The results showed that research activity in HTA varies considerably across Europe. The research was categorised into six areas: (1) the breadth of analysis in HTA (such as economic, organisational and social aspects); (2) HTA products developed to meet the needs of policy-makers (such as horizon scanning, mini-HTA, and core HTA); (3) handling life-cycle perspectives in relation to technologies; (4) topics that challenge existing methods and for which HTA should be developed to address the themes more comprehensively (such as public health interventions and organisational interventions); (5) development of HTA capacity and programmes; and (6) links between policy and HTA. An online survey showed that the three areas that were given priority were the relationship between HTA and policy-making, the impact of HTA and incorporating patient aspects in HTA. Policy-makers highlighted HTA and innovation processes as their main research priority. Areas that the systematic review identified as future priorities included issues within the six existing research areas such as disinvestment, developing evidence for new technologies, assessing the wider effects of technology use, and determining how HTA affects decision-making. In addition, relative effectiveness and personalised treatments are areas of growing interest. This study concluded that the research priorities identified are important for obtaining high quality and cost-effective health care in Europe. Managing the introduction, use and phasing out of technologies challenges health services throughout Europe, and these processes need to be improved to successfully manage future more general challenges. An ageing population and a diminishing workforce both require strong efforts to ensure effective and well-organized use of human resources and technologies. Furthermore, Europe needs to focus on innovation. This is closely linked to use of technologies and calls for future research (12).
Call for Research Proposals in New Methodologies for HTA
In 2011 the EU Framework Programme 7 shortlisted a number a project proposals to develop full proposals on the basis of a call for project proposals. This is a major step in bringing HTA into the research agenda in Europe.
Historical background to European HTA
Geographically, Europe includes approximately fifty countries with a total of approximately 730 million people. Politically, twenty-seven (twenty-eight with Croatia joining in 2013) of these countries (500 million people) have come together in the European Union. Governments are trying to on the one hand deal with issues of balancing pressures on public spending – which brings the health sector in a vulnerable situation – and on the other hand provide coherent, solidarity-based health and social systems (14). In order to balance biomedical advances with the resources available, the Governments of the European countries frequently turned to the scientific community to elucidate and explain what policy options there are to approach the challenges, and to provide some evidence as a basis for decisions of what ought to be publically funded (13).
In Europe, HTA started on a small scale in the 1970s. It grew throughout the 1980s and continues to do so (13). The European Commission, the executive branch of the European Union, supported several studies related to HTA in the early 1980s. These were mostly done under the program on health services research, and HTA was not seen strategically at that point in time, but was viewed as yet another form of health services research. However, by the mid 1980s, the Health Services Research Committee of the European Commission began to favour HTA with contracts on economic appraisal, variations in use of particular technologies, and mechanisms for regulating expensive health technologies in different countries (13). From 1993 to 2002 the European Commission supported and funded three projects to promote collaboration of Member States on HTA (5, 15, 16). However, there was no direct continuation of funding, and the network was discontinued after the third project had been completed.
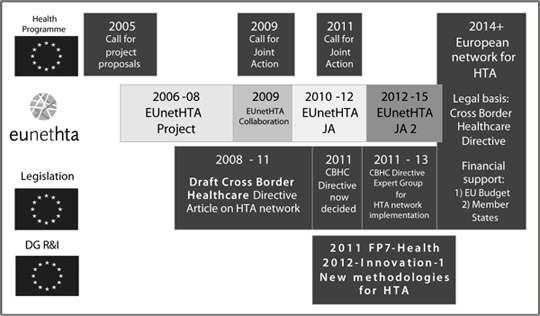
Figure. The time-line of reaching a sustainable and permanent HTA-network in Europe
Policy Background for the Emergence of a Sustainable Network for HTA in Europe
In 2002 a political process was started on cross-border health care by the Commission and the Health Council (Health Ministers from EU Member States). A report stated that HTA could assist policy makers in making informed decisions by providing evidence on medical, social, economic, and ethical issues concerning healthcare policy and practice (17). Work during 2004 resulted in a report that identified an urgent need to establish a sustainable network for HTA and proposed several steps starting with a 3-year project supported by the EU Public Health Program (2). The practice of HTA within this definition varies considerably across national settings. It informs policy and decision making in specific political, economic and institutional contexts. In order to be useful HTA has to be designed with processes and outputs that fit the relevant context (18). In July 2008 the European Commission issued a proposal for a directive on the application of patient rights in cross-border healthcare. An article of the proposal concerned cooperation on management of new health technologies, stating that Member States shall facilitate development and functioning of a network connecting the national authorities or bodies responsible for health technology assessment (19). In addition, discussion and conclusions on relative effectiveness assessment in the so-called Pharmaceutical Forum lead in the direction of HTA methodology (20). The Steering Committee of this Forum acknowledged that a EUnetHTA could take relative effectiveness assessment of pharmaceuticals forward. A Directive 2011/24 EU on the application of patients’ rights in cross-border healthcare was eventually put in place in March 2011. Article 15 specifies that The Union shall support and facilitate cooperation and the exchange of scientific information among Member States within a voluntary network connecting national authorities or bodies responsible for health technology assessment designated by the Member States (21).
Last Steps to Reach the Permanent Voluntary European Network for HTA with a Basis in European Legislation
The figure illustrates the parallel processes that lead to the materialisation of a permanent European network for HTA. In 2012 the European network is in the third and final year of a so-called Joint Action with the support of the EU Health Programme and a total budget of € 5.959.525 which will continue into a three-year Joint Action 2 with a budget of € 9.428.550 to start during the lasts months of 2012. In the duration of this action the Directive 2011/24 EU Article 15 HTA Network will be established and supported by the EU.
Literature
Velasco-Garrido M, Zentner A, Busse R: Health systems, health policy and health technology assessment. In: Velasco-Garrido M, Børlum Kristensen F, Palmhøj Nielsen C, Busse R (eds.) Health technology assessment and health policy-making in Europe – Current status, challenges and potential. Copenhagen: WHO Regional Office for Europe: 53–78, 2008.
Kristensen FB, Mäkelä M, Neikter SA, Rehnqvist N, Håheim LL, Mørland B, et al. Planning, development, and implementation of a sustainable European network for health technology assessment. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 84–91.
Kristensen FB, Lampe K, Chase D, Lee-Robin SH, Wild C, Moharra M, et al. Practical tools and methods for health technology assessment in Europe: structures, methodologies, and tools developed by the European Network for Health Technology Assessment, EUnetHTA. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 68–73.
Lampe K, Mäkelä M, Garrido MV Anttila H, Autti-Rämö I, Hicks NJ, et al. The HTA Core Model – A novel method for producing and reporting health technology assessments. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 1–8.
Banta D. Introduction to the EUR-ASSESS report. Int J Technol Assess Health Care 1997; 13: 133–143.
Turner S, Chase DL, Milne R, Cook A, Hicks NJ, Rosten C et al., The adaptation toolkit: Description and use. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 37–41.
Quentin F, Carbonneil C, Moty-Monnereau C, Berti E, Goettsch W, Lee-Robin SH. Web-based toolkit to facilitate European collaboration on evidence generation on promising health technologies. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 68–74.
Moharra M, EspallarguesM, Kubesch N, Estrada MD, Parada A, Vondeling H, et al. Systems to support health technology assessment (HTA) in Member States of the European Union with limited institutionalization of HTA. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 75–83.
Wild C, Simpson S, Douw K, Geiger-Gritsch S, Mathis S, Langer T. Information service on new and emerging health technologies: Identification and prioritization processes for a European Union–wide newsletter. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 48–55.
Nielsen CP, Lauritsen SW, Kristensen FB, Bistrup ML, Cecchetti A, Turk E; Involving stakeholders and developing a policy for stakeholder involvement in the European network for health technology assessment, EUnetHTA. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 84–91.
Håheim LL, Imaz I, Loud ML, Gasparetto T, González-Enriquez J, Dahlgren H, et al. Internal evaluation of the European network for Health Technology Assessment project. Int J Technol Assess Health Care 2009; 25 (Suppl 2): 99–106.
Nielsen CP, Funch TM, Kristensen FB. Health technology assessment: research trends and future priorities in Europe. J Health Serv Res Policy 2011; 16 (Suppl 2): 6–15.
Banta D, Kristensen FB, Jonsson E. A history of health technology assessment at the European level. Int J Technol Assess Health Care 2009; 25 (Suppl 1): 68–73.
Dalli J. Launching a discussion on the future HTA network. Member of the European Commission, responsible for heath and consumer policy, John Dalli’s speech at the EUnetHTA Conference in Gdansk, Poland, on December 8, 2011. http://ec.europa.eu/commission_2010–2014/dalli/docs/speech_08122011_en.pdf. Accessed December 21, 2011.
Banta HD, Oortwijn W. Conclusion: Health technology assessment and health care in the European Union. Int J Technol Assess Health Care 2000; 16: 626–635.
Jonsson E, Banta D, Henshall C, Sampietro-Colom L. Executive summary of the ECHTA/ECAHI Project. Int J Technol Asses Health Care 2002; 18: 213–217.
European Union. Health and Consumer Protection Directorate-General. High Level Group on Health Services and Medical Care. High level process of reflection on patient mobility and healthcare developments in the European Union. Outcome of the reflection process, 9 December 2003. Available at: http://ec.europa.eu/health/ph_overview/Documents/key01_mobility_en.pdf. Accessed December 21, 2011).
Børlum Kristensen F, Palmhøj Nielsen C, Chase D et al. What is health technology assessment? In: Velasco-Garrido M, Børlum Kristensen F, Palmhøj Nielsen C, Busse R (eds.) Health technology assessment and health policy-making in Europe – Current status, challenges and potential. Copenhagen: WHO Regional Office for Europe: 31–51, 2008.
Commission of the european communities. Proposal for a directive of the european parliament and of the council on the application of patients’ rights in cross-border healthcare, July 2, 2008. http://ec.europa.eu/health/ph_overview/co_operation/healthcare/docs/COM_en.pdf. Accessed December 21, 2011.
High Level Pharmaceutical Forum. Final Report, October 2, 2008. http://ec.europa.eu/pharmaforum/docs/ev_20081002_frep_en.pdf. Accessed December 21, 2011.
Directive 2011/24/eu of the european parliament and of the council of 9 March 2011 on the application of patients’ rights in cross-border healthcare http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:088:0045:0065:EN:PDF. Accessed December 21, 2011.
FBK@SST.DK
EUnetHTA Secretariat
National board of health
Islands Brygge 67
2300 Copenhagen S
Denmark
